Research Study: Regenerative Medicine for Sciatica Related to Disc Bulge or Disc Herniation
Regenexx and the Centeno Schultz Clinic in Broomfield, CO, are seeking study applicants with leg pain related to sciatica (lumbosacral radiculopathy) due to a disc herniation or bulge. Do I Qualify?Submit ApplicationWhat is the purpose of this study?
Regenexx® and the Centeno-Schultz Clinic are investigating whether sciatica patients receiving an epidural platelet lysate injection (which uses your own blood platelets) report better pain and functional outcomes compared to receiving an injection of saline.
How does the study work?
Participants who qualify will be randomly placed into one of two groups. You will not be aware of which group you are in for your initial treatment.
- Treatment Group: Receives a platelet lysate injection (made from their own blood).
- Control Group: Receives a saline injection (a placebo with no active treatment).
After the survey period is complete, participants will be compensated and those who initially received the placebo (saline) can elect to receive the real treatment at no cost.
What is platelet lysate and how could it help me?
The platelets in your blood contain multiple healing growth factors and exosomes. Approximately 150 randomized controlled trials have examined whether platelet-rich plasma can help chronic musculoskeletal and spine pain. The vast majority of these studies have been positive. Your platelets contain growth factors like NGF and BDNF (which can assist in nerve repair), TGF-b (which helps with tissue repair), and VEGF (which helps build new blood vessels). Epidural steroid injections are known to have negative side effects.
What will be measured?
Participants will complete surveys about their pain and symptoms before the injection and at 1, 2, 3, and 6 months afterward. This helps researchers understand if the treatment provides real benefits compared to a placebo.
What if I am in the Placebo Group?
Good news! You will be eligible to receive the platelet treatment at no charge, regardless of which group you start in.
Where is this study happening?
The study is conducted at the Centeno-Schultz Clinic in Broomfield, Colorado. Participants must visit the clinic for evaluation and treatment. As a clinic, CSC has used platelet lysate for two decades to help thousands of patients with disc herniations and bulges avoid surgery. In these procedures, blood is drawn, and then the growth factors in PRP are released to create platelet lysate, which is then injected using precise X-ray guidance into the area between the disc and the spinal nerve (epidural). The goal is to both reduce inflammation naturally and to heal the disc and nerve, thus reducing the need for surgery on the disc.
Who is a candidate for this study?
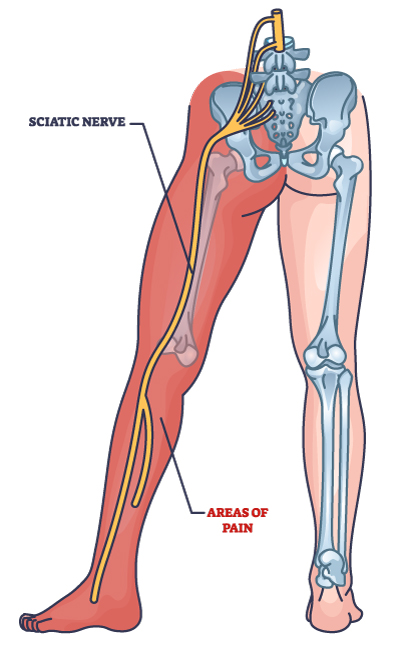
The ideal candidate lives within driving distance of Broomfield, CO. They experience leg pain in a single leg due to a lumbar spinal disc that is pressing on a nerve. This condition is commonly referred to as sciatica or lumbar radiculopathy. The ideal candidate will have MRI imaging verifying the condition. A summary of qualifiers is provided below, with more detailed inclusion and exclusion qualifications included in the toggle areas. If you have additional questions, please contact Ehren Dodson, PhD, Clinical Research Director using the contact form located here.
Who can participate?
To qualify, you must:
- Be between 18 and 65 years old.
- At least moderate pain due to sciatica lasting less than one year.
- Have tried treatments such as medication, physical therapy, chiropractic care, or exercise, without enough relief.
- Have imaging (MRI or CT scan) confirming sciatica that matches your symptoms.
Who cannot participate?
Unfortunately, you’re not eligible if you:
- Have had recent back surgery or an epidural injection within the last 2 months.
- Take medications like blood thinners, steroids, or opioids regularly.
- Have certain medical conditions like rheumatoid arthritis, depression, bleeding disorders, or if you’re pregnant.
- Have a history of drug abuse or allergies to study medications (lidocaine).
Detailed Inclusion Criteria
Candidates must meet ALL of the following:
- Must be 18-65 years of age, inclusive, at time of signing informed consent
- At least moderate pain at screening using Patient Global Impression of Severity (PGIS)
- Diagnosis of lumbosacral radiculopathy (LSR) radiating to or below the knee in a single dermatomal pattern (L4, L5, or S1) with onset of clinical symptoms less than 1 year prior to screening visit
- Presence of one of the following: a radicular pattern (L4, L5, S1) of sensory, reflex or strength changes
- Presence of persistent unilateral radicular pain. For participants with both leg and back pain, the leg pain must be worse than the back pain.
- LSR pain with inadequate response to conservative care (non-operative); participants must have tried at least one anti-inflammatory or analgesic medication (for at least 2 weeks at adequate doses) and at least one of the following: Physical therapy, bed rest, chiropractic manipulations, home directed lumbar and/or exercise programs.
- Lumbar spine MRI images are available after the onset of clinical symptoms and correlate with localization of clinical symptoms. CT is acceptable for patients with contraindication for MRI.
- Is independent, ambulatory, and can comply with post-treatment evaluations and visits.
- Voluntary signature of the IRB approved Informed Consent
Detailed Exclusion Criteria
Candidates will be excluded if they meet ANY of the following:
- Untreated underlying psychological conditions (e.g. depression, chronic pain syndrome, etc.) as a contributor of pain
- Bleeding disorders
- Currently taking anticoagulant or immunosuppressive medication
- Evidence on MRI or CT of recent vertebral fracture or segmental instability (spondylolisthesis)
- Inflammatory or auto-immune based pathology (e.g. rheumatoid arthritis, systemic lupus erythematosus, psoriatic arthritis, polymyalgia, polymyositis, gout, pseudogout, etc.)
- Co-existing hip or knee pain localized to the joint structures that may interfere with the participant’s ability to participate in the study or interfere with pain assessments
- Any central canal stenosis with neurogenic claudication (not including foraminal stenosis) with pain present mostly during walking and signs of lumbar stenosis on lumbar spine MRI/CT
- Has undergone a surgical procedure for back pain (e.g. discectomy, artificial disc replacement, fusion, etc)
- Presence of spinal cord stimulator
- Received epidural steroid injection or nerve blocks within the last 2 months
- Use of chronic opioids
- Documented history of drug abuse within the last 6 months
- Use of immunosuppressants, oral or intravenous steroids in the last 3 months
- Is pregnant
- Allergy or intolerance to study medication (e.g. lidocaine, etc.)
- Condition represents a worker’s comp case and/or is involved in a health-related litigation
- Presence of clinically significant disease that may interfere with the evaluation of safety and other clinical outcomes in the study
- Any other condition, that in the opinion of the investigator, would preclude the patient from enrollment
Yes, I think I meet the criteria!
Sciatica Study Application Form
If you have reviewed the qualification criteria above and would like to take the next steps, please complete the form below.
"*" indicates required fields